Inside Labs
Inside Labs
“Transporter” Protein Responsible for Zinc Influx in Cells Leads to Autoimmune Disease
Update 09.11.2022
The onset
and progression of rheumatoid arthritis, an autoimmune disease, is caused by
high zinc concentrations in immune cells, researchers report
Researchers
from Korea investigate the molecular dynamics that trigger rheumatoid arthritis
(RA). In their recent study, they demonstrate that an
increased influx of zinc in immune T cells, caused by an amplified expression
of zinc importer protein ZIP8 on their surfaces, contributes to the onset of
RA. On the brighter side, the researchers also report that controlling the
cellular zinc level through modulation of ZIP8 is a promising approach for the treatment
of RA.
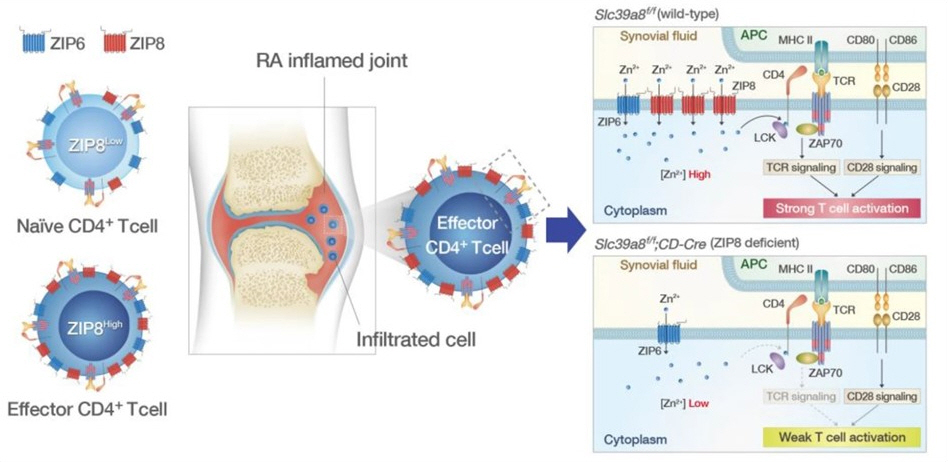
How ZIP8 protein modulation indirectly contributes to
rheumatoid arthritis
Researchers from Korea have recently reported the
effect of ZIP8 protein variation on cellular zinc influx and T cell activation,
which is found to be increased in rheumatoid arthritis.
Image courtesy: Sung-Gyoo Park from Seoul National
University.
Original source: Jung-Ah Kang et al. in Experimental
& Molecular Medicine, Springer Nature. Licensed under CC BY 4.0.
Rheumatoid arthritis (RA) is an autoimmune disease
characterized by inflammation of tissue and destruction of cartilage inside
joints and subsequent erosion of bones. Though the trigger for this disease is
not known, multiple studies on progression of the disease have revealed that
its onset and severity are associated with the cellular response of T-cells
(group of cells playing a vital role in the human immune system) including T
helper (Th) 17 cells. Hence, regulation of T cell responses is a promising
approach for amelioration of RA symptoms.
One of the possible methods for regulating immune
cell response is inducing a change in the cellular zinc composition of T cells.
Zinc is an essential trace element for normal functioning of immune response in
mammals. Balanced concentration of zinc in cells is maintained by two families
of transporter proteins: zinc exporter family (ZNT) and zinc importer family
(ZIP). While ZNT mediates the efflux of zinc from cells, ZIP mediates the
influx of zinc into cells. The ZNT family consists of 10 types of proteins
(ZNT1–ZNT 10) and the ZIP family consists of 14 types of proteins (ZIP1–ZIP14).
In previous studies, it was observed that expression of ZNT and ZIP varies
among different types of immune cells. However, the possibility that the cell
specific behavior of ZNT and ZIP and associated zinc movement within the cells
may be responsible for the onset of RA have hitherto remained unexplored.
To explore this possibility, a group of researchers
from Korea, led by Prof. Sung-Gyoo Park of Seoul National University, investigated
the functioning of ZNT and ZIP families in immune cells in mice models of RA.
The investigation revealed significantly high concentration of zinc in T cells
present in joint fluid and joint tissues in RA mice models. Upon checking the
activities of ZNT and ZIP proteins to analyze the increased zinc level in T
cells in joint tissues, the researchers found the ZIP8 protein to be
hyperactive. Almost 99.8% of T cells expressed ZIP8 on their cell surface
leading to an increased influx of zinc. The results of their study, which was
supported by a research grant from Korea Centers for Disease Control and
Prevention (grant number 2020-ER6902-00), have recently been published in Experimental & Molecular Medicine.
Explaining the significance of their finding, Prof.
Park states, “Zinc is essential for the maintenance of the immune system,
which is why many people take zinc supplements. However, our study indicates
that an increase in cellular zinc concentration in T cells caused by
hyperactivity of zinc transporter ZIP8 protein contributes to the onset and
exacerbation of RA. This led us to examine whether modulation of ZIP8 protein
is able to reduce cellular zinc level and symptoms of RA.”
For this purpose, the researchers examined the role
of ZIP8 in T cell receptor (TCR; proteins on T cell surface, which help
identify antigens) signaling and found that ZIP8 deficiency dramatically
reduced zinc influx in T cells, thereby reducing TCR signaling. Attenuation of TCR signaling further leads to
weakening of T cell activation and response. This proves that targeting
transporter proteins can be an effective way of regulating zinc levels in
immune cells and treating autoimmune diseases.
Prof. Park concludes by surmising, “Our complex immune system is affected by various factors, such as food, stress, and the environment. It is not entirely possible, nor recommended to treat autoimmune diseases caused by imbalance of cellular zinc solely through diet. It is necessary to understand the molecules responsible for the imbalance and target them for treatment. Hence, in our study, we examined the role of ZIP8 in the development of RA and also showed that modulating them can be an appropriate treatment option for RA. Perhaps within the next 5 to 10 years, we will be able to develop technology to modulate zinc transporter proteins and treat T cell-mediated inflammatory diseases.”
This certainly bodes well for people suffering from
RA, and other autoimmune disorders, across the world.
Reference
Authors Jung-Ah
Kang1,2,3, Ji-Sun Kwak1,4, Sang-Heon Park1,2,5,
Kyu-Young Sim1,2,5,
Seul Ki Kim1,4, Youngnim Shin1,4, In Jung Jung1,4, Jeong-In Yang1,4, Jang-Soo Chun1,2,4 and Sung-Gyoo Park1,2,5
Title of original paper ZIP8 exacerbates collagen-induced arthritis by increasing pathogenic T cell responses
Journal Experimental
& Molecular Medicine
DOI 10.1038/s12276-021-00591-1
Affiliations 1School of Life Sciences, Gwangju Institute of Science and Technology, Gwangju 61005, Republic of Korea
2Cell Logistics Research Center, Gwangju Institute of Science and Technology, Gwangju 61005, Republic of Korea
3Infectious Disease Research Center, Korea Research Institute of Bioscience & Biotechnology (KRIBB),
Daejeon 34141, Republic of Korea
4National Creative Research Initiatives Center for Osteoarthritis Pathogenesis, Gwangju Institute of Science and Technology,
Gwangju 61005, Republic of Korea
5College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea
About National Institute of Health in Korea
The Korea National Institute of Health (KNIH), one
of the major operating components of the Ministry of Health and Welfare, leads
the nation’s medical research. Over the past seven decades, the KNIH has made
unwavering efforts to enhance the public’s health and innovate biomedical
research. The KNIH seeks to eradicate diseases and make people healthier. The
KNIH establishes a scientific basis and evidence underlying health policy as
well as provides national research infrastructures. We also promote public
health research. To this end, we make efforts to enrich a health research
environment by granting funds to research projects and keeping our resources,
data, and facilities more open and accessible to researchers.
Website: http://www.nih.go.kr/eng/
About Professor Sung-Gyoo Park
Prof.
Sung-Gyoo Park received his PhD in Molecular Biology in 2003 from the Seoul
National University. Thereafter, he completed his postdoctoral training from
Prof. Sankar Ghosh’s lab at the Yale University. He then tenured as a Professor
in the School of Life Sciences at the Gwangju Institute of Science and
Technology. Currently, he is with the Seoul National University as a Professor
in the College of Pharmacy. Prof. Park’s research group is involved in
development of approaches for controlling inflammatory diseases by identifying
the responsible molecules. They also focus on inflammatory diseases, especially
those involving central nervous system, which are caused due to infections.

